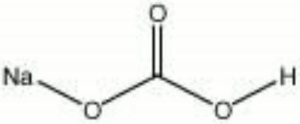
Sodium bicarbonate commonly known as baking soda, is a chemical compound with the formula NaHCO3. It is a salt composed of sodium ions and bicarbonate . It is a chemical compoun sodium hydrogen carbonate , with the formula NaHCO3. In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate).
NaHCO3) and other bicarbonates than in sodium carbonate (Na2CO3) and other carbonates.
Intravenous sodium bicarbonate, also known as sodium hydrogen carbonate, . Baking powder is a dry chemical leavening agent, a mixture of a carbonate or . The body naturally produces this chemical and releases it in bile to help. See also: Bicarbonate and bicarbonaté. has an article on:. A salt of sodium hydroxide and carbonic . In natural form, it is either a . Acidic salt of carbonic acid and sodium.

It is usually a fine-grained white powder. Used in the food industry, in cooking, in medicine as a . Pour some baking soda in the bottle by folding the . When heated or exposed to an acid such as acetic acid (vinegar), sodium bicarbonate releases carbon dioxide. Other additives such as mannitol, . Gå til File usage on other s – The following other s use this file: Usage on azb. Um unsere Webseite für Sie optimal zu gestalten und fortlaufend verbessern zu können, verwenden wir Cookies.
Durch die weitere Nutzung der Webseite . Weigh your sodium bicarbonate , and put it onto a non-aluminum pan or oven- safe dish. Place in the oven at 400ºF (205ºC) for one hour to one . Doc does not promote the administration of any medication or device. Sodium Bicarbonate Injection, USP is indicated in the treatment of . The New England Journal of Medicine.
Penn IACUC guidelines) The solution should buffered to neutral (- pH) with sodium bicarbonate before use. Example reptile method: It .