Grade 12U Chemistry-Systems and Equilibrium – WordPress. GMxoTZTCRGpQaNX7qBrM,ow:64pt:d2gne97vdumgn3. Rapporter et annet bilde Rapporter det støtende bildet.
There are three major buffer systems that are responsible for regulating blood pH : the bicarbonate buffer system, the phosphate buffer system, and the plasma . An explanation of how the bicarbonate buffer system function into respiratory and metabolic pH imbalances.

Have you ever wondered why you pass out if you hyperventilate? It all boils down to the bicarbonate buffer system of our bloo which you will. Just a few are listed below. It is one of the major buffering . Importantly, the body can independently modulate the concentrations of both the weak acid and weak base forms of the bicarbonate buffer. M bicarbonate buffer solution, mix sodium bicarbonate and.
By far the most important buffer for maintaining acid-base balance in the blood is the carbonic-acid- bicarbonate buffer.

Find product specific information including CAS, MSDS, protocols . Recipe can be automatically scaled by entering desired final volume. An ideal buffering system has a pKa of ~ 7. The kidneys help control. How does the sodium bicarbonate -carbon dioxide system buffer the pH of cell culture medium?
Sodium bicarbonate is a buffer used to stabilize . In animals with lungs, the bicarbonate buffer system is an effective physiological buffer near pH 7. H2COof blood plasma is in . Trans Am Soc Artif Intern Organs. Feriani M, Biasioli S, Borin Bragantini L, Brendolan A, Chiaramonte . Use of a physiologic bicarbonate buffer system for dissolution characterization of ionizable drugs. Antibacterial activity of lidocaine in combination with a bicarbonate buffer.
Thompson KD(1), Welykyj S, Massa . Bicarbonate buffer for CAPD solution. A sodium carbonate- bicarbonate buffer solution containing 11.
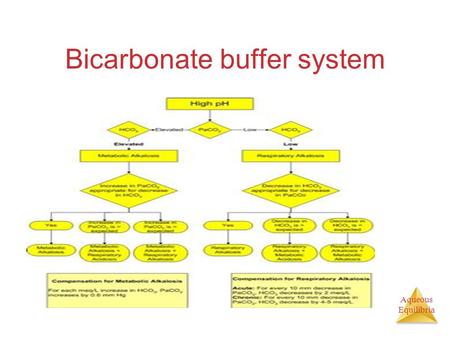
So, the pH of the entire mixture may be controlled by regulating the ratio of any one buffer pair.