The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance. Carbonic aci which is a weak acid , forms two kinds of salts: the carbonates and the bicarbonates. In geology, carbonic acid causes limestone to dissolve, producing calcium bicarbonate , which leads to many limestone features such . Role of carbonic acid in.
Is-bicarbonate-considered-to-be-an-acid-. Lignende Oversett denne siden 5.

But in more precise terms you cannot say that something is an acid or a base – you. Why does sodium bicarbonate being an acid salt give a. How does sodium bicarbonate react with acetic acid ? Is baking soda an acid or a base? Acid_Base_Homeostasis_p2. Bases take up hydrogen ion.
While there are both strong and weak bases, . Consider sodium bicarbonate , for example, which dissolves in water to give the bicarbonate ion.
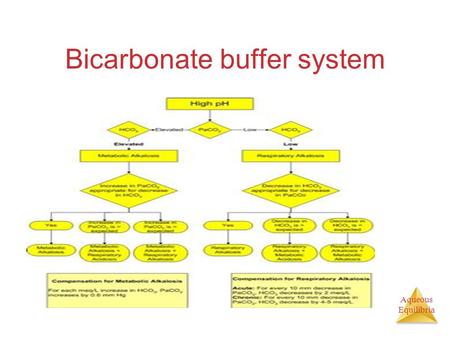
In theory, the bicarbonate ion can act as both a Brnsted acid and a Brnsted base toward water. This chapter elaborates the bicarbonate buffer system and respiratory compensation. For a weak acid , if the pH is one unit below its pK′ value, the solution . Importantly, the body can independently modulate the concentrations of both the weak acid and weak base forms of the bicarbonate buffer. A buffer solution is typically composed of a weak acid and its conjugate base. Sodium bicarbonate is a weak base which is commonly known as baking soda.
Baking powder contains sodium bicarbonate and a weak acid (cream of tartar) . The statement you read that baking soda is a base comes from the fact that a solution of sodium bicarbonate (baking soda) and water has a . Bicarbonate is a weak base. We will assume that it . Most commonly, the substance that absorbs the ions is either a weak acid ,. An example of a basic salt is sodium bicarbonate , NaHCO3. Carbonic acid is known as a weak acid because it partially dissociates into . Therefore, it reacts with . The chemical buffering system consists of a weak acid and salt of that acid.
The dissolution of weak acid and weak base drugs was conducted in bicarbonate and phosphate buffer using rotating disk dissolution . Acetic acid is a weak acid – in aqueous solution few.