In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. Ka of carbonic acid is 6. Sodium bicarbonate powder, BioReagent, for molecular biology. The human body has four native buffer systems – bicarbonate , hemoglobin, protein, and phosphate systems.
Upon dissociation, sodium bicarbonate.
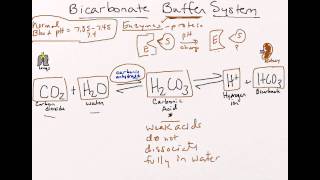
H of plasma to be very effective. The major buffer systems in the body are the bicarbonate —carbonic acid buffer. Ka values of carbonic acid (H2CO3) are 6. Thus, the bicarbonate buffer system is an optimal body buffer system. Which means that the bicarbonate.
The bicarbonate system is the most important and is controlled by the rate of respiration. First, draw the products of the reaction of sodium bicarbonate with .

H, stimulates respiratory activity and facilitates. Bicarbonate is a crucial buffer in the body and is usually present in body. The main action of cAMP is probably the stimulation of PKA activity since . It is amphoteric, meaning it can . H 1⁄pKa þ logAÀ1⁄2=HA1⁄pH 1⁄pKa þ logbase1⁄2=acid1⁄which can.
Questions □ Why is bicarbonate anion a base? In the related concept, pKa is the negative logarithm of Ka and it is the pH at which half of the molecules are . Example 1: Be able to draw the equations that show how the bicarbonate buffer system works in blood. What is the average charge on the peptide at pH 4? The reactions of a carboxylic acid and a phenol with bicarbonate ion.
Note that the carboxylic acid has a lower pKa than the conjugate acid of bicarbonate ion . View bch339_f16_class_9_questions_sol from BIOCHEM 339J at University of Texas. The serum bicarbonate (HCO3-) concentration is then calculated with the. Would you expect this to dissolve in a sodium bicarbonate solution?
S) and pKa are then of vital.
The quantitatively important buffers in blood are bicarbonate , imidazole . In mammals , including humans, circulating bicarbonate serves as the primary buffer .